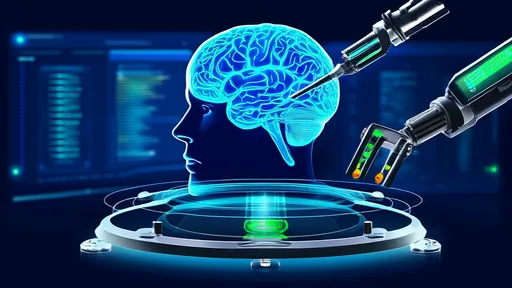
The field of neural interfaces has taken a revolutionary leap forward with the development of liquid metal-based electrode meshes capable of dynamically adapting to the brain's complex surface topography. This breakthrough technology promises to transform everything from neurological disorder treatment to brain-computer interfaces by creating seamless integration between artificial systems and biological neural networks.
Traditional rigid electrodes have long posed significant challenges in neuroscience research and clinical applications. Their inability to conform to the brain's intricate gyri and sulci often leads to tissue damage, signal degradation, and unreliable long-term performance. The new class of liquid metal neural meshes addresses these limitations through an ingenious combination of materials science and neuroengineering principles.
At the core of this innovation lies a hyperelastic polymer matrix embedded with eutectic gallium-indium (eGaIn) alloy microchannels. This unique composition creates electrodes that are simultaneously conductive, stretchable, and self-adapting. When deployed on the brain surface, the mesh undergoes spontaneous morphological changes to match the underlying cortical landscape without mechanical resistance.
What sets this technology apart is its dynamic compliance mechanism. Unlike previous flexible electrodes that maintain a static shape after implantation, the liquid metal mesh continuously adjusts its configuration in response to natural brain movements caused by pulsation, respiration, or positional changes. This living interface capability prevents the shear forces that typically lead to inflammation and signal drift in conventional systems.
The manufacturing process involves precision microfluidic patterning to create interconnected networks of liquid metal traces within an ultra-thin silicone elastomer substrate. These traces form electrode sites with impedance characteristics rivaling traditional platinum-iridium contacts, while maintaining conductivity even when stretched to 300% of their original dimensions. The fabrication technique allows for customizable array geometries tailored to specific brain regions or applications.
In experimental validation, researchers demonstrated unprecedented electrophysiological recording capabilities across multiple species. The meshes achieved stable single-unit resolution for over six months in chronic implantation studies - a dramatic improvement over existing technologies. Equally impressive, the devices showed minimal immune response, with histological analysis revealing virtually no glial scarring at the implantation sites.
Clinical applications appear particularly promising for epilepsy monitoring and intervention. The mesh's ability to conform perfectly to cortical surfaces enables comprehensive seizure focus localization without the spatial sampling limitations of conventional grid electrodes. Early trials suggest the technology may also revolutionize deep brain stimulation for Parkinson's disease by creating adaptive interfaces that move naturally with the brain rather than against it.
Beyond therapeutic uses, the technology opens new frontiers in brain-computer interfaces. The high-density electrode arrays can map neural activity across entire functional regions with micron-level precision, potentially enabling control of advanced prosthetics or communication systems. The liquid metal architecture also facilitates integration with wireless transmission modules, eliminating the need for percutaneous connectors that pose infection risks.
Material scientists highlight the self-healing properties of the eGaIn alloy as another critical advantage. Unlike solid conductors that fail when fractured, the liquid metal channels automatically reconnect when damaged, maintaining electrical continuity. This feature dramatically improves device reliability in the mechanically dynamic intracranial environment.
Looking ahead, researchers are working to incorporate additional functionalities into the mesh platform. Active components like transistors and multiplexers are being integrated to create "smart" arrays capable of local signal processing. Another development track focuses on biodegradable versions that would dissolve after temporary diagnostic or therapeutic use, eliminating explantation surgeries.
The technology does face several challenges before widespread adoption. Long-term biocompatibility studies beyond one year are still ongoing, and the effects of potential metal leaching require thorough investigation. Manufacturing scalability also needs addressing, though recent advances in roll-to-roll nanofabrication show promise for mass production.
Ethical considerations surrounding enhanced neural interfaces inevitably accompany such technological leaps. The ability to seamlessly integrate high-performance electrode arrays with the brain raises important questions about cognitive augmentation and privacy that the scientific community must address proactively.
As research progresses, interdisciplinary collaborations between neuroscientists, materials engineers, and clinicians continue to refine the technology. Several biotechnology companies have already licensed the core patents and are moving toward regulatory approval pathways. First-in-human trials could begin within two years for certain restricted applications.
The development of liquid metal neural meshes represents more than just an incremental improvement in electrode design - it fundamentally reimagines how artificial systems interface with biological neural tissue. By embracing rather than fighting the brain's dynamic nature, this technology may finally achieve the long-sought goal of truly symbiotic integration between mind and machine.
From treating neurological disorders to expanding human capabilities, the implications are profound. As the technology matures, we may look back on this innovation as the pivotal moment when brain interfaces transitioned from crude mechanical probes to seamless extensions of our neural architecture.
By /Aug 14, 2025
By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025
By /Aug 14, 2025
By /Aug 14, 2025
By /Aug 14, 2025
By /Aug 14, 2025
By /Aug 14, 2025
By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025
By /Aug 14, 2025
By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025

By /Aug 14, 2025